-
 Physical and chemical properties of PET as packaging plastic
Physical and chemical properties of PET as packaging plastic -
 Physical and chemical properties of PET as packaging plastic
Physical and chemical properties of PET as packaging plastic -
 Physical and chemical properties of PET as packaging plastic
Physical and chemical properties of PET as packaging plastic
Updated at
2023-07-22 11:33:41

Physical and chemical properties of PET as packaging plastic
Thermal behavior
One of the most important characteristics of polymers is their thermal behavior. The knowledge related to this behavior is necessary not only for choosing the right process and manufacturing conditions, but also for identifying the physical and mechanical properties as well as choosing the right materials for various applications. The properties of polymers have the most changes at two specific temperatures. This temperature is the melting temperature of crystals (TM) for crystalline polymers and the glass transition temperature (Tg) for amorphous polymers.
The preform/PET bottle shows three transfer points, it is important to know these values for these containers:
Glass transition temperature (Tg)
Crystallinity (crystallization) temperature under which the polymer crystallizes a small amount.
The melting temperature at which the crystal structure is destroyed.
Differential Scanning Calorimetry (DSC) is a technique for measuring parameters such as Tg, melting temperature and crystallinity percentage.
Many properties of semi-crystalline polymers such as fragility, optical transparency, barrier properties (ability to prevent the transfer of gases), mechanical resistance, retention time, thermal stability, etc. depend on the values of these parameters. Therefore, calculating these values for preforms and PET bottles is particularly important.
Most polymers that are completely amorphous, or even crystalline polymers that have a small amount of amorphous region, are rigid and glassy below a temperature called the glass transition temperature Tg.
At temperatures above the glass transition temperature and at least at slow to moderate rates of deformation, amorphous polymers are soft and flexible.
Many physical properties change rapidly upon reaching the glass transition temperature. These properties include the coefficient of thermal expansion, thermal coefficient, refractive index, mechanical loss, nuclear magnetism, electron spin resonance behavior, electrical properties, tensile strength, and the final amount of deformation in elastomers.
Tg can be considered as the most important property of polymer materials in the investigation of mechanical properties.
The glass transition temperature of polymers is very important in terms of their inhibitory properties against gases, and in this way it affects the durability and quality of the packaged material. The temperature range at which a polymer can act as a good barrier against gases is related to the Tg of that polymer. The higher the glass transition temperature, the better it can act as a coating and inhibitor in a wider temperature range.
At temperatures higher than the glass transition temperature, the mobility of chains and empty spaces increases, and as a result, the permeability increases, but at temperatures lower than that, the polymer is glassy and hard, and its permeability is low.
When converting the glass form of the polymer to the rubber form at temperatures higher than the glass transition temperature, the disorder, free volume, and mobility of the macromolecules that make up the polymer increase.
Crystallization behavior
Crystalline means that the polymer chains are parallel and close together, and amorphous means that the polymer chains are irregular. Most polymers are arranged in complex structures, consisting of crystalline and amorphous regions.
In the bottle manufacturing process, crystallinity usually results from heating above the glass transition temperature (Tg) and is often accompanied by molecular orientation.
The degree of crystallinity depends on the inherent properties of the polymer as well as on external factors. The molecular weight range, linear chain structure and high molecular weight are very important prerequisites to obtain more crystallinity.
The crystallinity is affected by external factors such as stretch ratio, single or biaxial stretch type and temperature in the bottle production stage.
Below the glass transition temperature, the polymer chain is rigid. After reaching the glass transition temperature, the chains are more flexible and able to open under pressure. If stretching is performed at a temperature above the glass transition temperature, the randomly entangled and disordered shape of the chain begins to unravel and begin to rearrange, some of them even sliding onto the nearest neighboring chain.
Crystallinity in PET is usually caused by thermal crystallization or by stress and strain. Thermal crystallization occurs when the polymer is heated above its glass transition temperature and is not cooled quickly enough. In this case, the polymer becomes opaque due to the spherulitic structure.
In stress-induced crystallization, stretching or orientation is applied to the hot polymer, and the chains rearrange in parallel in planes close to each other. Orientation means forcing the material out of a non-oriented state by a tensile force. For example, pasta in a container has a pattern similar to a polymer chain, which is placed in a network pattern by the tension rod and the force of the tail air.
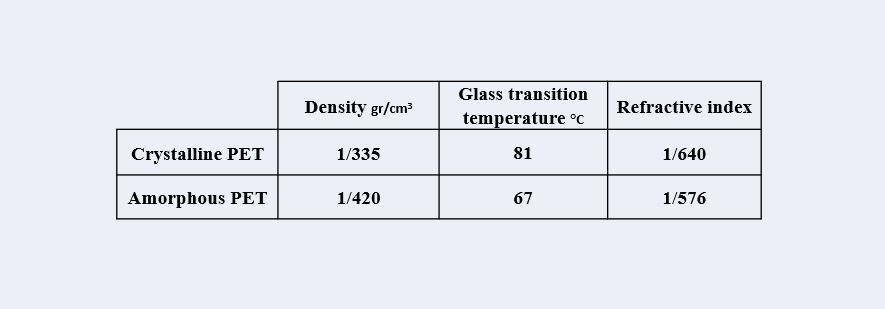
Polyethylene terephthalate (PET) is a polymer with the ability of crystallization due to the order of its chemical and geometric structure. The degree of crystallinity of PET depends on its thermal history. Crystalline PET melts at a temperature of about 255 degrees Celsius and turns into an amorphous form if cooled quickly (density 1.33 g/cm3)
Crystallization begins at a temperature of about 80°C due to slow molecular movements, and at a temperature of 130°C, the density will reach 1.37 g/cm3. With proper orientation and heat application, the density reaches 1.37 g/cm3.
The degree of crystallinity and morphology of PET significantly affects the properties of the polymer.
Polymers with higher crystallinity, higher glass transition temperature (glass transition temperature for amorphous PET is 67°C and for crystalline PET is 81°C), higher modulus, toughness, stiffness, tensile strength, hardness and More solvent resistance, but less impact resistance.
The mechanical properties and optimal transparency of a bottle depend on the relationship between crystallinity and axial orientations.
In fact, the superiority of PET materials in the blow molding process depends on three characteristics of these materials:
crystallinity,
Crystal speed is slow
strain hardening
When PET is stretched in the stretch-blow molding process, it reaches a point beyond which it requires more force to stretch, called the natural stretch ratio (NSR).
This point is observed for materials that have a hard strain phenomenon on their stress-strain curve.
The phenomenon of hard strain needs more investigation, which is briefly mentioned.
When the temperature of the PET preform is between the glass transition temperature (Tg) and its crystallization temperature, strain hardening occurs.
The behavior of PET against stretching is shown in the stress-strain curve.
Amorphous PET is stretched uniaxially at a temperature between Tg and the crystallization temperature:
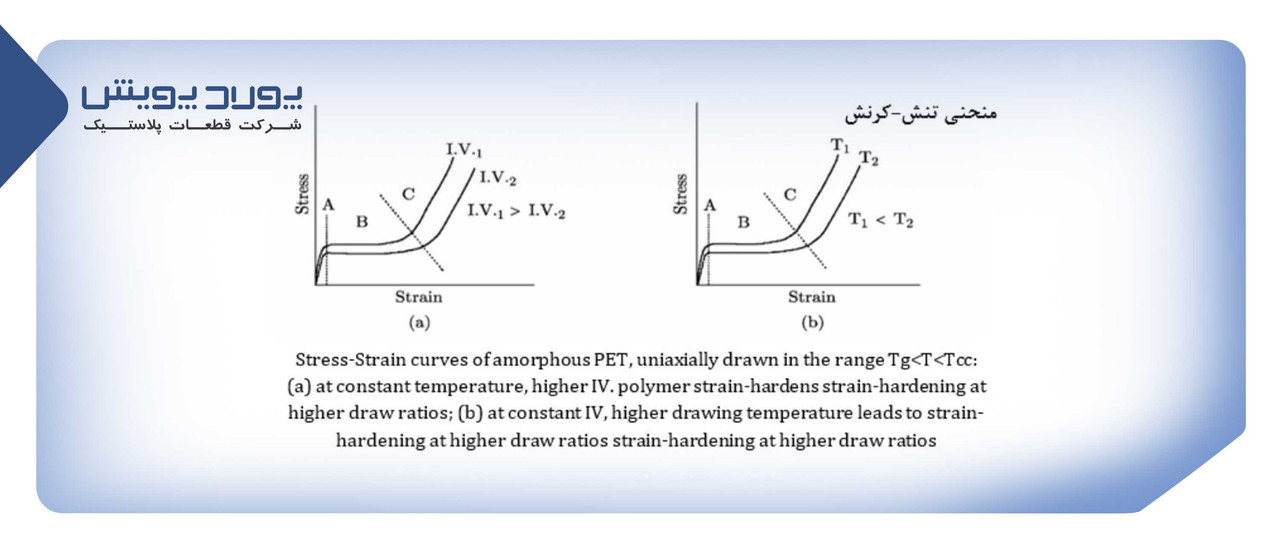
The stress resulting from the division of force per unit area and strain is the ratio of changes in the length of the sample to its initial length.
As seen in the PET strain stress curve, after the elastic region (region A), the polymer is stretched at a constant stress (ie region B). The characteristics of the B region or the normal stretching region depend on the intrinsic viscosity (IV) and temperature. Lower viscosity and higher temperature cause strain hardening at higher strain ratios.
In area C, despite the reduction of the cross-sectional area, due to the increase in crystallinity and the alignment of polymer chains, the strength increases up to 5 times, this phenomenon is called hard strain, so in this area, PET needs more force to be stretched.
The hard strain phenomenon does not occur in polymers such as polyvinyl chloride (PVC) or polycarbonate, which do not have the ability to crystallize. In fact, it is the phenomenon of hard strain that has made it possible to use the stretch blow molding process in the production of PET bottles. The hard strain prevents the tearing of the thinner areas during the stretching of the denser areas of the preform, as well as the uniform distribution of the material in the bottle.
Chemical resistance
Chemicals can affect the plastic's flexibility, surface finish, color, dimensions, and weight.
These changes can be caused by the following factors:
Chemical attack on the polymer chain: reactions such as oxidation, reaction of functional groups and degradation of the polymer (which can cause a decrease in the physical properties of the polymer).
Physical changes such as solvent absorption by the polymer and as a result its softening and swelling, solvent leakage from the plastic and dissolution in the solvent are other factors affecting the chemical resistance of the polymer, temperature, pressure and internal and external stresses, exposure time, concentration of chemicals and environmental cracking stress.
Environmental stress cracking is the failure of a plastic material in the presence of certain types of chemicals. This failure is not the result of a chemical attack.
Three factors affect cracking stress:
Tensile strength, a cracking stress factor and the inherent susceptibility of plastics to cracking stress. The alignment of these three factors can cause this problem.
Crack-causing stress factors are found in detergents, surface-active chemicals, grease, oil, ultrapure water, and additives such as polishes and wetting agents. In some cases, a relatively low concentration of a cracking stress agent may be sufficient to cause cracking.
It should be noted that the reaction of compounds from two or more different groups may create another chemical substance that strengthens or weakens the favorable or unfavorable effect of each compound.
Note that before testing the desired substance in a specific package, consider the safety points related to it, including the possibility of flammability.